

Affiliations
ABSTRACT
Pulmonary sequestration is a rare congenital anomaly of the lung. It presents as an area of dysplastic and non-functional pulmonary tissue, with aberrant blood supply and multiple pathways of venous drainage. It usually presents in the left lung and is supplied by a single branch from the thoracic aorta. We, herein, report an interesting case of this uncommon condition, diagnosed and managed at our hospital. The patient was diagnosed after multiple episodes of pneumonia, using a computer tomography angiography (CTA), with a right lung pulmonary sequestration that had a dual blood supply from the thoracic aorta. He underwent excision via biportal video-assisted thoracoscopy (VATS). There were multiple challenges intra-operatively which included the presence of multiple enlarged lymph nodes, dense adhesions, and isolation of the right-sided dual blood supply from the thoracic aorta safely. The patient had an uneventful postoperative recovery.
Key Words: Pulmonary sequestration, Congenital abnormalities, Thoracoscopy.
INTRODUCTION
Pulmonary sequestration is a rare congenital anomaly. It is a dysplastic sequestered lesion that is disconnected from the remaining tracheobronchial tree.
Congenital pulmonary airway malformations are seen in 1 per 8,300 to 35,000 patients. Pulmonary sequestrations comprise 0.15 to 6.4% of all congenital lung malformations.1 Given the rarity of this condition, we found it prudent and essential to report the case of this 21-year male patient, who was diagnosed with right-sided pulmonary sequestration with dual blood supply from the thoracic aorta that was resected via minimally invasive approach in the setting of dense adhesions from previous recurrent episodes of pneumonia.
CASE REPORT
A 21-year male presented to the emergency room (ER) with right-sided pleuritic chest discomfort and upper respiratory tract infection (URTI) symptoms. The patient was a heavy smoker of electric cigarettes for three years.
Upon examination, his vital signs were normal. His chest examination revealed diminished right lower chest breath sounds.
ER chest radiographs indicated right lower lobe opacity. Computer tomography angiography (CTA) of the chest revealed a right lower lobe soft tissue lesion with an uneven border and punctate calcifications. Two arterial branches (Figure 1) from the descending thoracic aorta supplied the lesion caudal to the inferior pulmonary vein. An infection caused a right lower lobe 7.5 mm solid lung mass. The diagnosis of infected right-sided intralobar pulmonary sequestration was suggested.
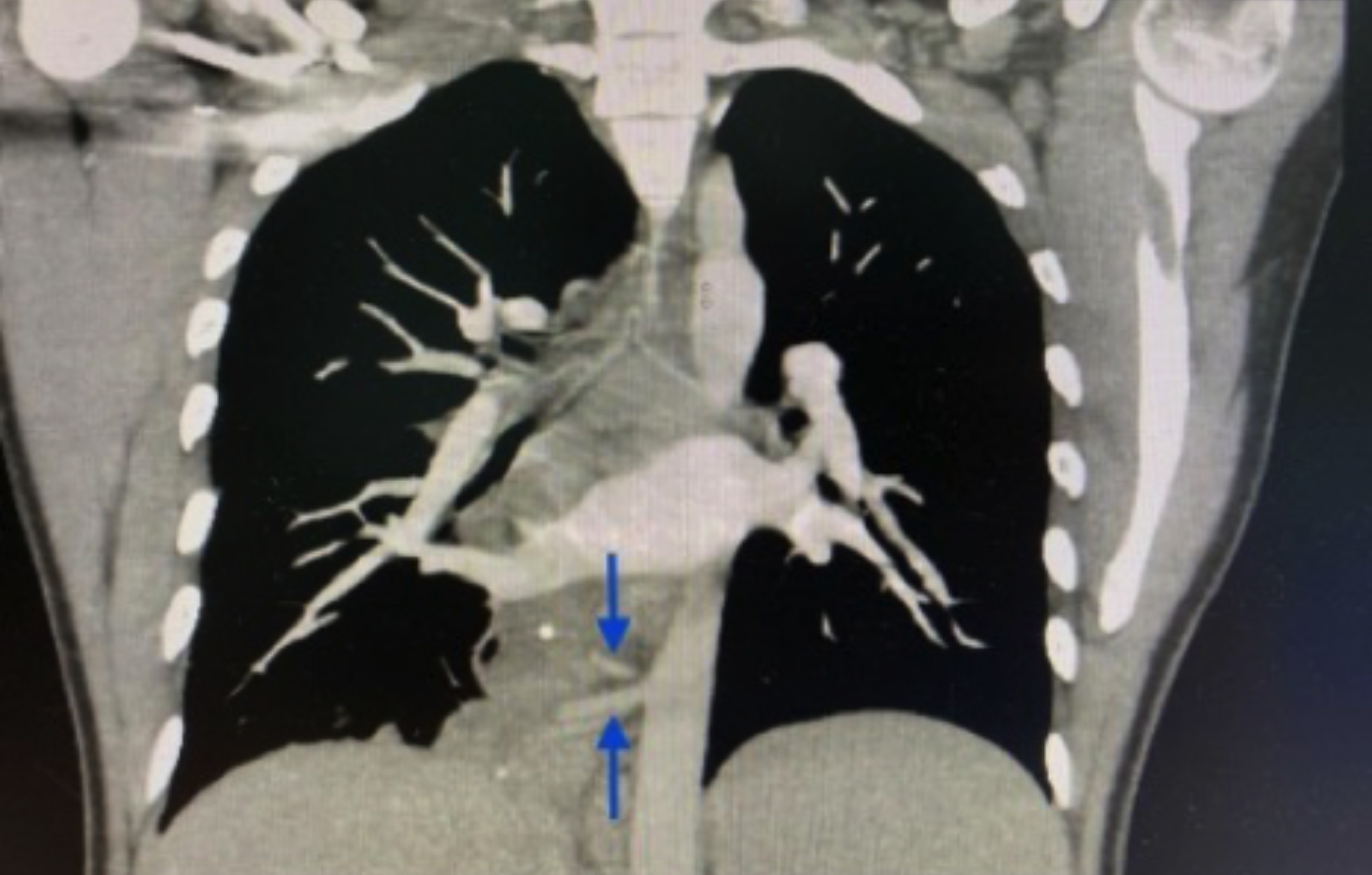 Figure 1: Dual arterial supply branches (arrows) reaching the mass from the aorta.
Figure 1: Dual arterial supply branches (arrows) reaching the mass from the aorta.
The patient received intravenous antibiotics during his five-day hospital stay. After six weeks of treatment, the infection resolved, and he was scheduled for resection. The patient underwent right lower lobectomy and lymph node sampling by biportal thoracoscopy with a camera port, that was used for the chest tube insertion. The LigaSure was used to dissect both aortic branches feeding the mass intraoperatively. Vascular staplers were used to divide both branches (Figure 2). Then, we proceeded with the standard right lower lobectomy. There were multiple enlarged lymph nodes and dense adhesions surround-ing the arterial and bronchial branches.
Since malignancy was not excluded, mediastinal lymph node sampling from stations 4, 7, and 9 was done.
The patient was discharged on the third postoperative day. Histopathology confirmed the diagnosis.
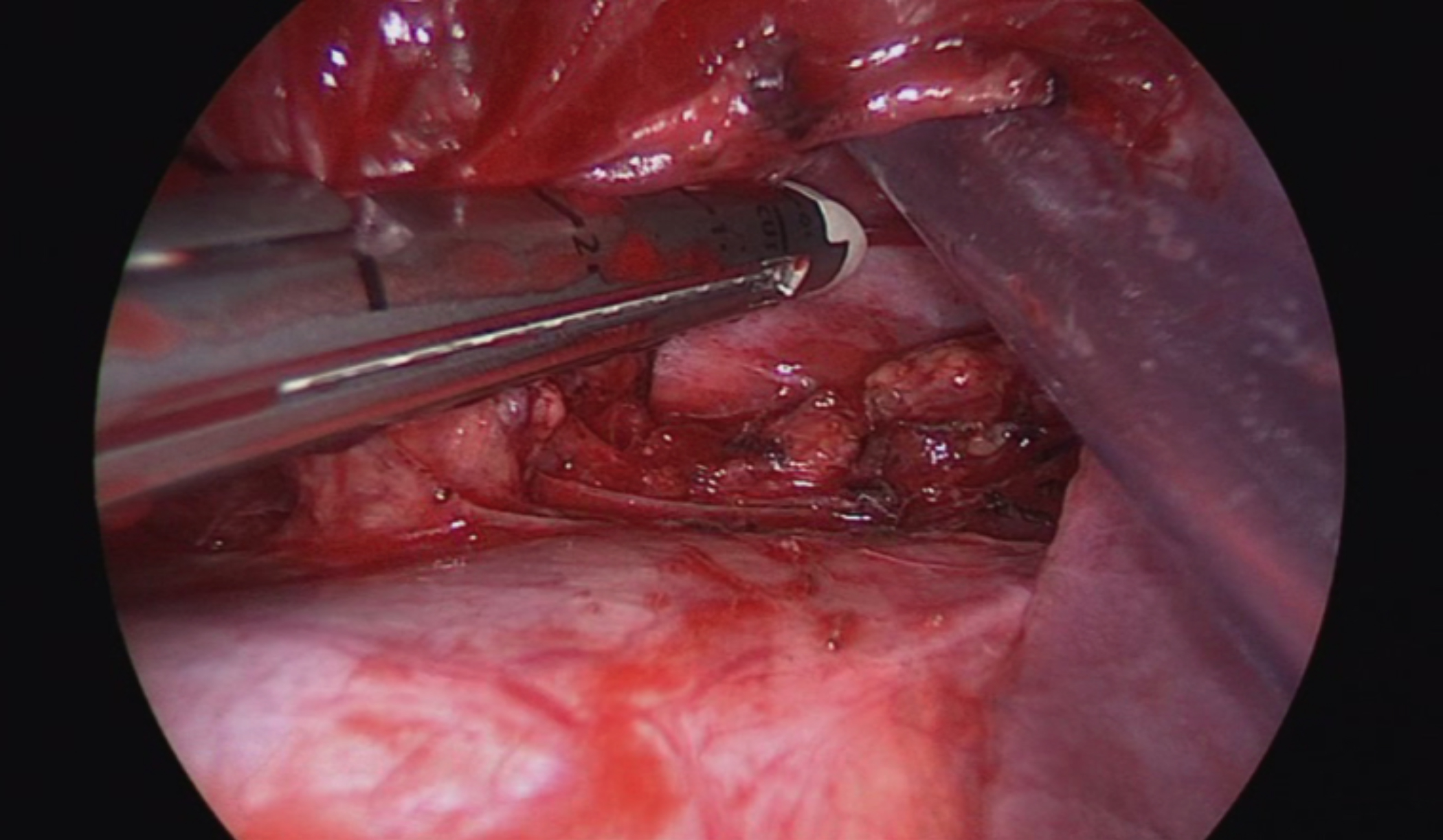 Figure 2: Two individual branches of the descending aorta (arrows) were identified and divided.
Figure 2: Two individual branches of the descending aorta (arrows) were identified and divided.
DISCUSSION
Pulmonary sequestration is a rare congenital lung anomaly. It presents as an area of dysplastic and non-functional pulmonary tissue, with aberrant blood supply and multiple pathways of venous drainage. The dysplastic sequestered lesion is disconnected from the remaining tracheobronchial tree.1
CTA of the chest helped us to achieve a confirmatory diagnosis in this patient. As demonstrated by the previous studies, CTA of the chest is an appropriate and optimum tool for confirmatory diagnosis of pulmonary sequestration.2
The right-sided location of the pulmonary sequestration in this patient was less commonly found compared to the left-sided lesions as shown in a large retrospective analysis of 2,625 cases of pulmonary sequestration, in which the location of the lesion was reported in 2,037 cases. The analysis revealed that 71.53% lesions were localised in the left lobe while the right lobe was less frequently affected, with merely 25.7% right-sided lesions being reported. This large-scale study demonstrated that right-sided pulmonary sequestrations have a nearly three-fold lower occurrence than the left-sided ones.3
A review of 540 published cases of pulmonary sequestration by Savic et al. showed that the ratio of left-sided versus right-sided pulmonary sequestration is around 60:40.4
An exception among pulmonary sequestrations are the ones associated with Scimitar syndrome (partial anomalous pulmonary venous connection). These abnormalities are often accompanied by right-sided intralobar pulmonary sequestration.5,6 However, in the present patient, no such abnormality was detected.
The vascular supply of pulmonary sequestration was quite peculiar. In the majority of sequestrations, the aberrant blood supply arises from the thoracic aorta. In a large retrospective review, Wei and Li reported data of arterial supply of 1,808 cases of pulmonary sequestration, which showed blood supply to >75% lesions originating from the thoracic aorta (1,384 cases, 76.55%).3
However, what added to the rarity of this case was the dual arterial supply from two individual branches of the descending thoracic aorta. Wei and Li, in their review, reported the number of arteries supplying the lesions in 813 cases.3 Of these, nearly 80% lesions were supplied by a single artery whereas dual arterial supply was seen in merely 15.9% of the cases.
A surgical intervention is mandatory for treating pulmonary sequestration. Often patients present with acute respiratory symptoms that warrant the need for surgery. Even in asymptomatic cases, the clinical consensus has been in favour of elective surgery, as untreated pulmonary sequestration carries a significant risk of recurrent respiratory tract infections, severe haemoptysis, haemothorax, and development of lung neoplasms. Hence, upon diagnosis, elective surgery for pulmonary sequestration is recommended.7
Thoracoscopic lobectomy for the treatment of pulmonary sequestration is associated with less blood loss (p=0.032), a greater drainage volume (p=0.001), and a longer chest tube duration (p=0.001) compared to thoracotomy. Thoracotomy and thoracoscopy have a comparable safety index.8
For this patient, neither wedge resection nor segmentectomy seemed appropriate. In the right lower lobe, he had a sizable sequestration with undefined irregular borders.
Right-sided pulmonary sequestration with abnormal dual arterial supply was an uncommon finding. These lesions usually form dense adhesions and enlarged lymph nodes around them secondary to recurrent pneumonia. Hence, resection via minimally invasive approaches is less likely to be utilised in these patients. We believe that this uncommon pathology and the surgical approach utilised to manage it will contribute to the previously limited body of evidence on this condition, particularly from Saudi Arabia. We recommend thoracoscopic lobectomy for large sequestrations as the most effective and safest surgical approach. Pulmonary sequestration must always be considered in the differential diagnosis of lung masses.
PATIENT'S CONSENT:
Written, informed consent was obtained from the patient.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SK: Drafting and revising the work, and writing the manuscript.
AA: Literature review, writing the manuscript.
SA, WH: Drafting and revising the work.
All authors approved the final version of the manuscript to be published.
REFERENCES